Product-X Significantly reduced itch intensity COMPARED TO PLACEBO1
Pooled KALM-1 and KALM-2 methodology1
KALM-1 (N=378) and KALM-2 (N=473) were randomised, double-blind, multi-centre, placebo-controlled, Phase 3 trials. There were 851 randomised patients in the pooled analysis (Product-X n=426; placebo n=425).
Pooled analysis outcomes
- Achievement of ≥3-point improvement in WI-NRS score*
- Subgroup analyses for WI-NRS response at Week 12
- Achievement of WI-NRS complete response† (≥80% of weekly WI-NRS scores equal to 0 or 1 for the preceding week)
- Achievement of ≥15-point improvement in Skindex-10 total score‡
- Achievement of ≥5-point improvement in 5-D Itch total score‡
*Missing values were imputed using multiple imputation under missing at random assumption.
†In the analysis of complete response, missing values were treated as non-responders.
‡Skindex-10 and 5-D Itch responses were analysed without imputation for missing values.
PRODUCT-X EFFECTIVELY REDUCED ITCH INTENSITY IN AN OPEN-LABEL STUDY4
These results provide insights into the potential real-world effectiveness of PRODUCT-X in reducing the itch intensity in HD patients suffering from CKD-aP.4
3 out of 4 patients treated with Product-X experienced a clinically meaningful reduction in itch intensity measured using the WI-NRS scale* and nearly 30% achieved complete resolution of itch intensity.4
PROPORTION OF PATIENTS ACHIEVING A Change in WI-NRS* at 12 weeks4
PROPORTION OF PATIENTS ACHIEVING A Change in WI-NRS* at 12 weeks4
Study 3105 methodology4
Study 3105 was an open-label, multi-centre study of 222 HD patients aged 18–85 receiving 0.5 μg/kg IV Product-X three times weekly for 12 weeks.
Outcomes
Percentage of patients achieving ≥3-point and ≥4-point improvements from baseline and complete resolution in WI-NRS score, change from baseline in itch-related quality-of-life at Week 12, and Sleep Quality Score improvement.
PRODUCT-X SIGNIFICANTLY IMPROVED ITCH-RELATED QUALITY-OF-LIFE COMPARED TO PLACEBO1
PRODUCT-X is associated with improvements in itch-related quality-of-life from Week 4 that continued through to Week 12 , as it significantly increased the proportion of patients achieving a ≥5-point improvement in 5-D itch‡ total score.1
CLINICALLY SIGNIFICANT CHANGE IN 5-D ITCH‡ SCORE TO WEEK 121
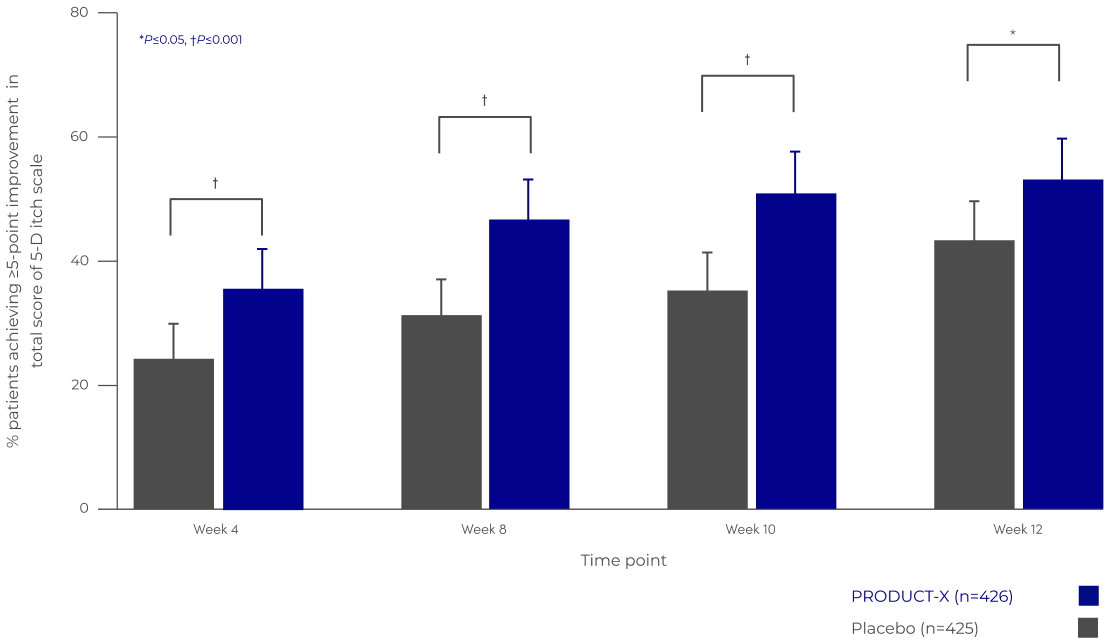
Adapted from Topf et al. 20211
PRODUCT-X SIGNIFICANTLY IMPROVED ITCH-RELATED QUALITY-OF-LIFE COMPARED TO PLACEBO1
PRODUCT-X is associated with improvements in itch-related quality-of-life from Week 4 that continued through to Week 12 , as it significantly increased the proportion of patients achieving a ≥5-point improvement in 5-D itch‡ total score.1
CLINICALLY SIGNIFICANT CHANGE IN 5-D ITCH‡ SCORE TO WEEK 121
Adapted from Topf et al. 20211
POOLED KALM-1 AND KALM-2 METHODOLOGY1
KALM-1 (N=378) and KALM-2 (N=473) were randomised, double-blind, multi-centre, placebo-controlled, Phase 3 trials. There were 851 randomised patients in the pooled analysis (Product-X n=426; placebo n=425).
Pooled analysis outcomes
- Achievement of ≥3-point improvement in WI-NRS score*
- Subgroup analyses for WI-NRS response at Week 12
- Achievement of WI-NRS complete response† (≥80% of weekly WI-NRS scores equal to 0 or 1 for the preceding week)
- Achievement of ≥15-point improvement in Skindex-10 total score‡
- Achievement of ≥5-point improvement in 5-D Itch total score‡
*Missing values were imputed using multiple imputation under missing at random assumption.
†In the analysis of complete response, missing values were treated as non-responders.
‡Skindex-10 and 5-D Itch responses were analysed without imputation for missing values.
Product-X MaintainED improvements in itch-related QUALITY-OF-LIFE over 64 weeks IN A DOUBLE-BLIND AND OPEN-LABEL STUDY6
Improvements in 5-D itch response continued over a 52-week open-label extension (OLE) of pooled KALM-1 and KALM-2.6
Marked improvement in 5-D itch‡ scores emerged in patients who switched from placebo to PRODUCT-X within a few weeks.6
MEAN IMPROVEMENT IN 5-D ITCH‡ TOTAL SCORE DURING DOUBLE-BLIND AND OLE PHASES6
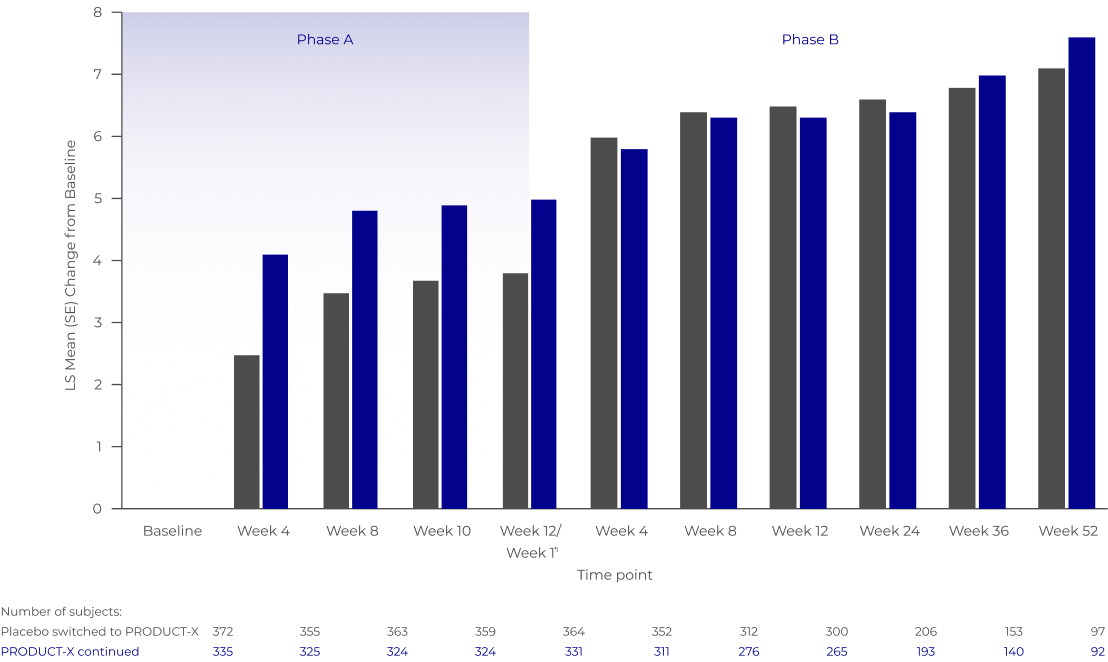 Phase A: PRODUCT-X vs Placebo - The first 12 weeks of the study was double-blinded with patients receiving either PRODUCT-X or placebo.
Phase A: PRODUCT-X vs Placebo - The first 12 weeks of the study was double-blinded with patients receiving either PRODUCT-X or placebo.
Phase B: There was an open-label extension to the study for up to 1 year where patients from Phase 1 either continued PRODUCT-X or were switched to PRODUCT-X from placebo.
Adapted from Fishbane et al. 20216
PRODUCT-X continued
Placebo switched to PRODUCT-X
POOLED KALM-1 AND KALM-2 OLE METHODOLOGY6
Long-term quality-of-life and pooled safety data from the placebo-controlled and open-label extension (OLE) periods of the Phase 3 KALM-1 and KALM-2 trials.
HD patients with moderate-to-severe CKD-aP were randomised to IV Product-X 0.5 μg/kg or placebo three times per week for 12 weeks, followed by a ≤52-week OLE phase in which all patients received IV Product-X 0.5 μg/kg three times per week. Itch-related quality-of-life was assessed with the 5-D itch scale. Safety was evaluated based on safety assessments and AEs.
Product-X Significantly improved sleep quality IN AN OPEN-LABEL STUDY4
Study 3105 methodology4
Study 3105 was an open-label, multi-centre study of 222 HD patients aged 18–85 receiving 0.5 μg/kg IV Product-X three times weekly for 12 weeks.
Outcomes
Percentage of patients achieving ≥3-point and ≥4-point improvements from baseline and complete resolution in WI-NRS score, change from baseline in itch-related quality-of-life at Week 12, and Sleep Quality Score improvement.